Orthocell has been granted a licence by the Food and Drug Administration of Thailand to commence sales of Remplir™ into the significant and growing US$84 million Thai nerve repair market.
Orthocell Appoints its First Four US Remplir™ Distributors
Orthocell has appointed four US distributors for its flagship nerve repair product Remplir™, enabling the commencement of commercial distribution into the US.
AusBiz | Interview with John Van Der Wielen
Orthocell Chair John Van Der Wielen spoke with Ausbiz about the Company’s ramp up in the US.
Orthocell Receives Regulatory Approval for Striate+ in Brazil
Orthocell has announced that Brazilian Health Regulatory Agency, ANVISA, has granted regulatory approval for the Company’s market-leading dental membrane, Striate+™, allowing the Company to commence sales in the large and important market of Brazil.
MEDIA | 7 News
Orthocell’s recent FDA clearance made headlines on 7 News this week, with journalist Alice Murray showcasing the human impact of our nerve repair technology.
MEDIA | 9 News
Orthocell welcomed 9 News journalist Michael Stamp to Orthocell HQ last week to film an in-depth segment about our FDA clearance for the nightly news.
US FDA grants 510(k) clearance for Orthocell’s flagship Remplir™ nerve repair product
Orthocell has announced that the US Food and Drug Administration has granted regulatory clearance under the 510(k) pathway for the Company’s nerve repair product, Remplir™.
First Sales of Striate+™ in DACH Region
Orthocell has today announced that our exclusive global distribution partner BioHorizons has officially completed first sales of Orthocell’s dental product, Striate+™, in Germany, Austria and Switzerland.
Orthocell Receives Regulatory Approval for Striate+ in Singapore
Orthocell has received regulatory approval from Singapore’s Health Sciences Authority (HSA) for its market leading dental guided bone regeneration product, Striate+™.
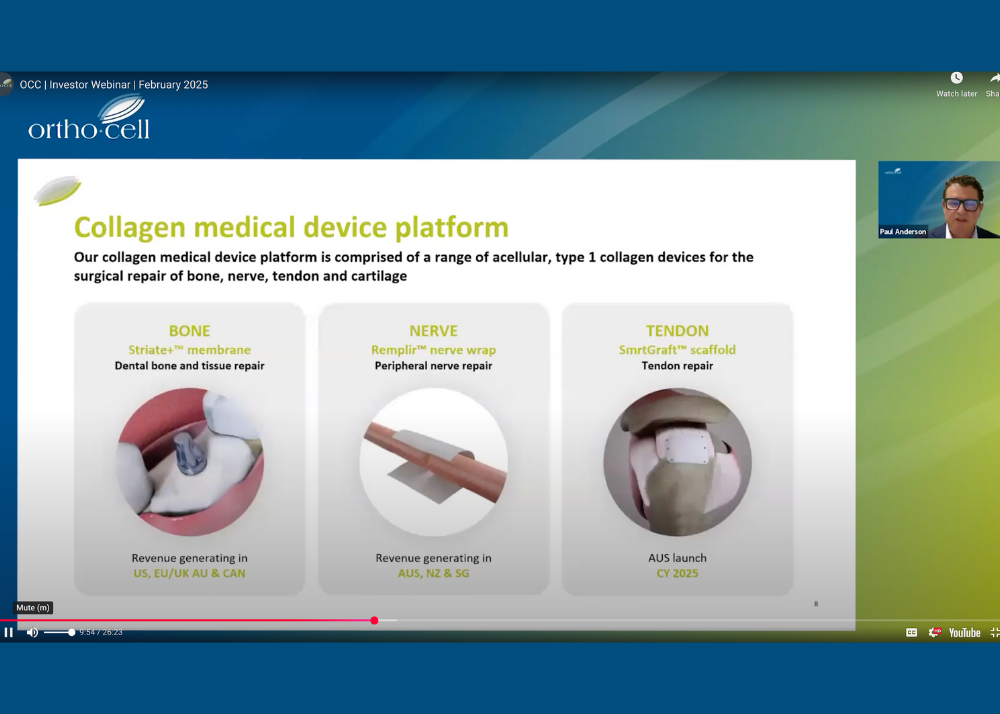
REPLAY | Investor Webinar
Orthocell CEO and MD, Paul Anderson, hosted a webinar this morning to provide an important update on Orthocell’s clinical and commercial plans.
Watch the replay: