Orthocell is a regenerative medicine company dedicated to the development of breakthrough products for the treatment of musculoskeletal disorders.
CelGro™ Platform Collagen Medical Device
Collagen medical devices are designed to augment surgical repair of bone and soft tissue. Collagen medical devices are manufactured using a proprietary SMRT™ manufacturing process to preserve the natural collagen structure. The devices are completely absorbed by the body and can be used alone or combined with cell therapies to augment the repair of bone, nerve, tendon and cartilage defects.
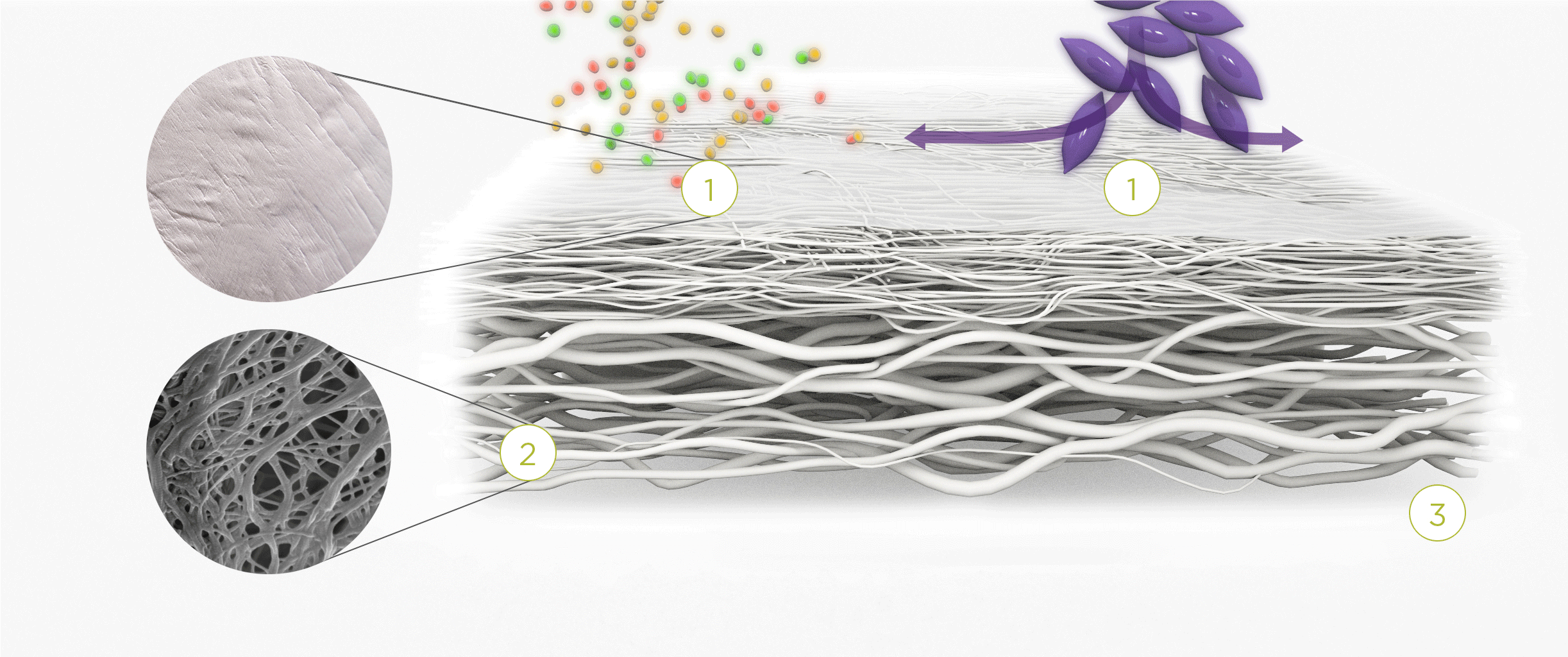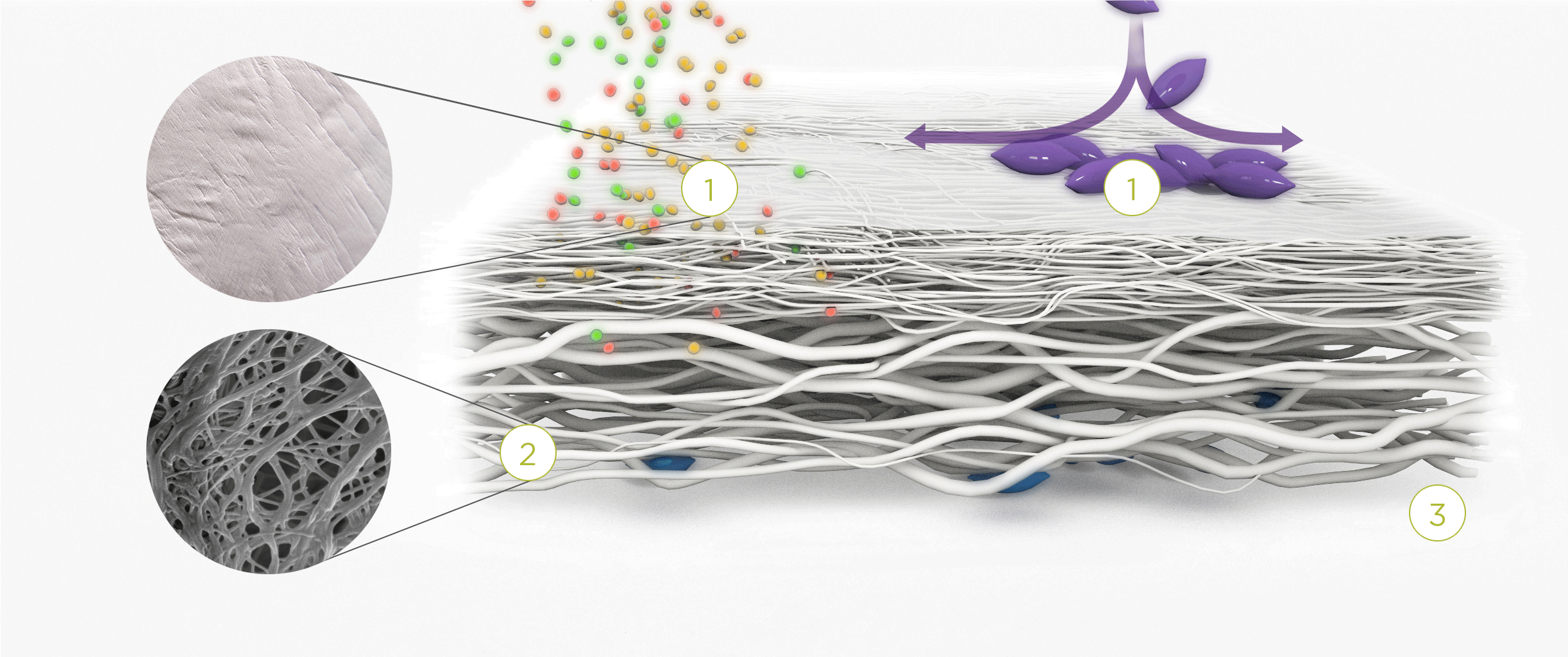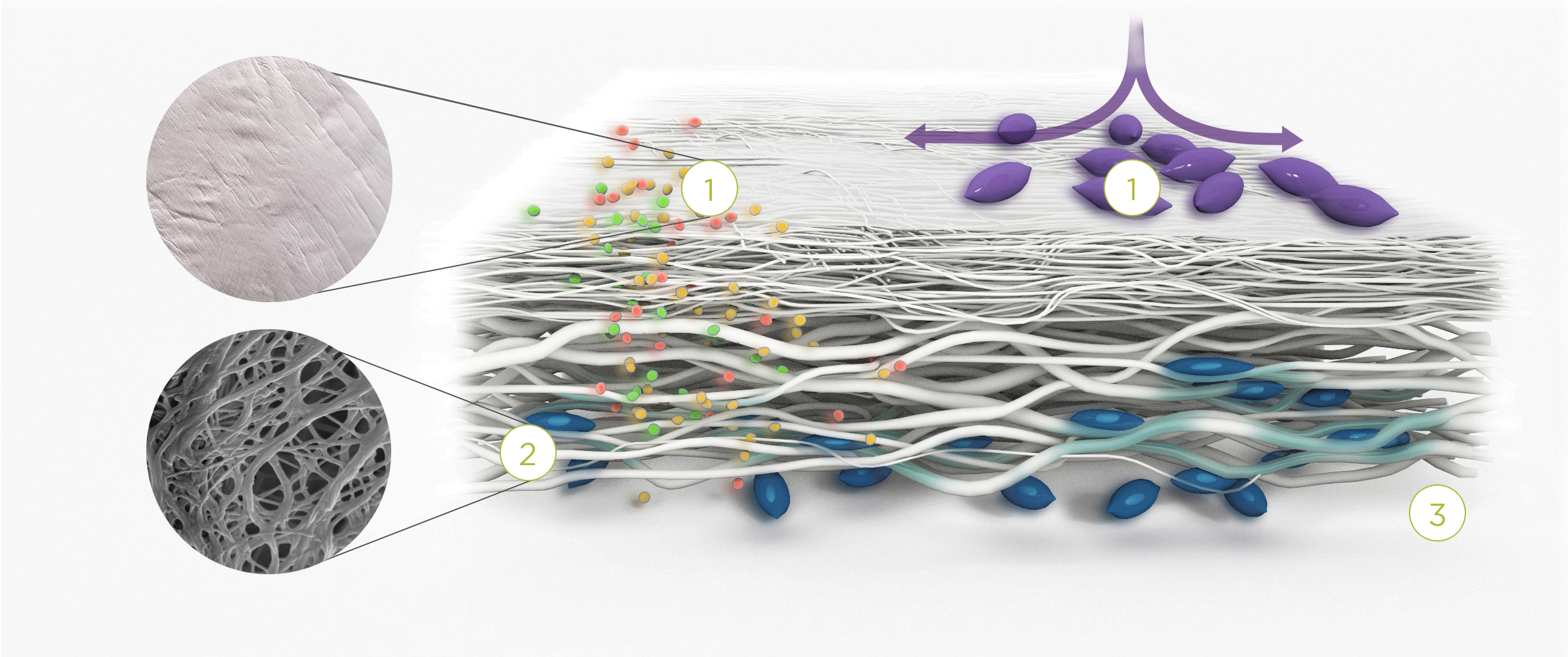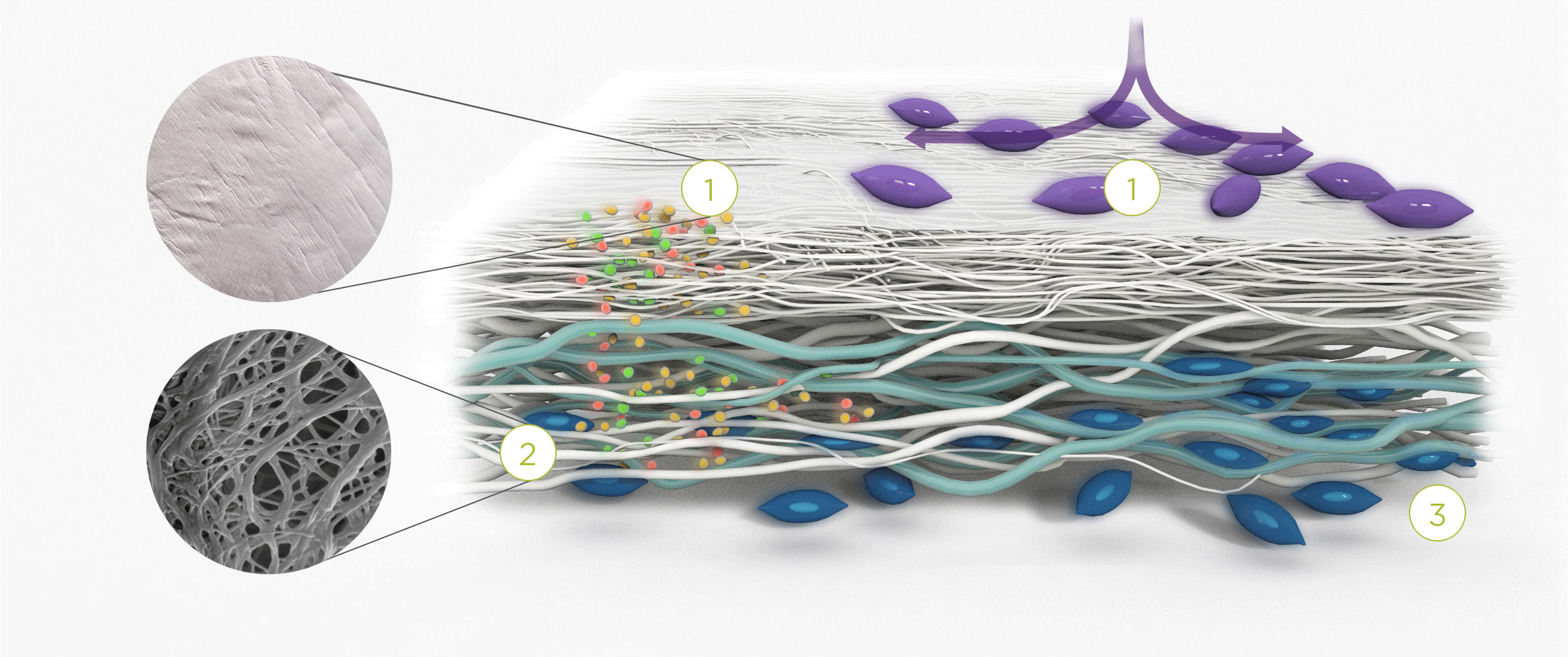
1. | 2. | 3. |
| The membrane prevents infiltration of cells and enables the passage of bioactive molecules and proteins | Porous network of collagen fibers promotes cellular attachment and proliferation | Bioactive chamber supports new tissue formation and integration of the membrane at the repair site |
How it works
The Collagen medical devices’ natural bilayer structure, which features smooth and rough sides, acts to both guide new tissue formation and protect the repair site.
Smooth layer – a parallel arrangement of densely packed collagen bundles with abundant pores permits the passage of fluids and nutrient molecules while preventing the invasion of external tissue into the site of repair.
Rough layer – consists of loosely arranged collagen bundles to provide a scaffold to support new cell growth.
Tissue remodeling – cells from the regenerating tissue integrate with the collagen fibres to guide tissue regeneration.
Cell Therapies
Cell therapy aims to treat diseased or damaged tissue by local implantation or injection of healthy cells where tissue repair is needed. The use of a patient’s own cells (autologous) to repair tissue damage reduces the risk of rejection or transmission of infectious diseases.
Innovative treatment for chronic tendon injuries
Orthocell’s autologous tendon therapy is the most advanced injectable cellular therapy for the treatment of chronic tendon injuries. The patient’s own healthy tendon derived cells are used to assist in the regeneration of damaged tendon to reduce pain and return function. The innovative therapy addresses a significant unmet clinical need for a safe, effective, and non-surgical solution. Orthocell is at the clinical stage of development for this innovative cell therapy with more than 1,000 patients treated.
Regeneration of human cartilage
OrthoACI™ (Autologous Chondrocyte Implantation) offers treatment for symptomatic cartilage defects of the knee and ankle. Damaged cartilage has a limited capacity for self-repair and if left untreated, the cartilage damage may progress to the point where joint replacement is required. OrthoACI™ uses the patient’s own healthy cartilage cells, called chondrocytes, to assist in the regeneration of cartilage tissue. OrthoACI™ is available for use in Australia.
Our Founding Story
Orthocell’s origins lay in a purposeful meeting of bright, solutions-focused minds more than 15 years ago, who saw the potential for biological solutions to repair the human body, in applications traditionally dominated by materials like metal and plastic.
One of those leaders was Orthocell’s Co-Founder and Managing Director, Paul Anderson, a former orthopedic nurse who moved swiftly into the commercial side of medicine to work in product sales and ultimately, as a successful entrepreneur, working alongside respected academic visionaries in the orthopedics space.
Through his association with The University of Western Australia, Paul started collaborating with Orthocell’s now Co-Founder and Chief Scientific Officer, Professor Ming Hao Zheng, to develop technologies to produce novel regenerative medicine treatments.
Theirs is a strong and proven partnership. Paul and Professor Zheng have previously commercialized cartilage repair technology together – and sold it to an American company – which had a current market capitalization of more than US $1B. Key learnings from this chapter challenged them to pursue the next ‘big thing’ in regenerative medicine.
With two promising products in the pipeline, they secured venture capital funding from a Western Australian-based VC firm to build the laboratory and fund the clinical trial programs; together with non-diluted funding from the State government. Orthocell was listed on the Australian Securities Exchange (ASX) in 2014.
World-Class Manufacturing Facility
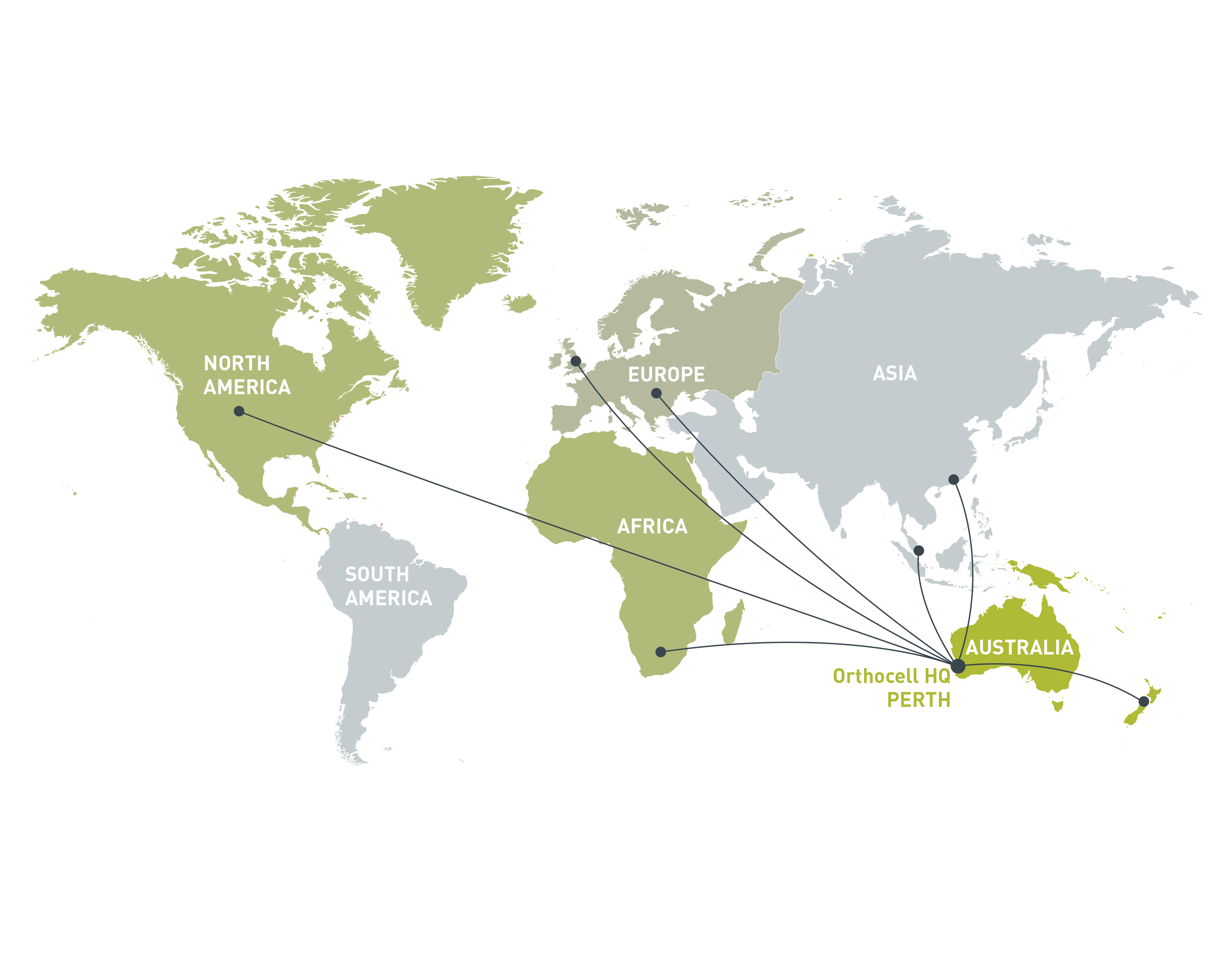
Orthocell has established a quality-controlled facility at its headquarters in Perth, Australia. The facility is licensed by the Therapeutic Goods Administration (TGA) for the manufacture of human tendon cells (tenocytes) and cartilage cells (chondrocytes) for the regeneration of damaged tendon and cartilage. The facility is also certified to ISO 13485 for the manufacture of CelGro™. Orthocell is equipped to manufacture, scale up and export products around the world.