Orthocell (ASX $OCC) has today released an update on its Ortho-ATI™ rotator cuff tendon study – the database is now locked and the Company is track to report the highly anticipated top line results in Q4 this calendar year.
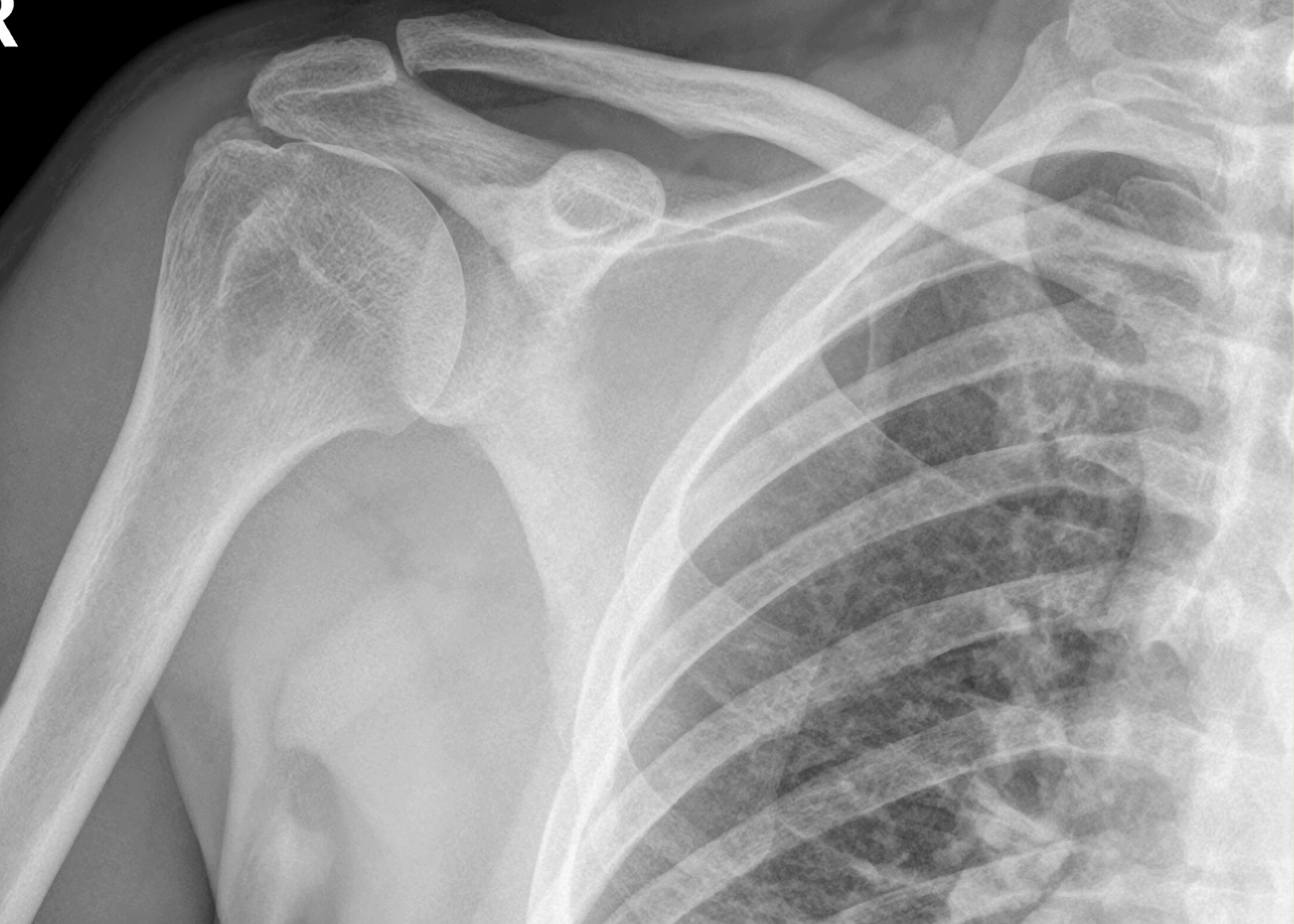
This study was designed to assess the effectiveness of Ortho-ATI™, compared to corticosteroids, as a non-surgical treatment for a complex, hard-to-treat clinical problem.
Should the study meet its objectives, Orthocell is well positioned to deliver the first FDA approved injectable cell therapy in orthopaedics for the treatment of chronic tendon injuries in a rotator cuff market estimated at around US$2.8b per annum.