Orthocell’s Striate+™ dental membrane, which facilitates high quality and efficient bone and tissue repair, is gaining commercial momentum.
Meet Orthocell’s Quality Director, Monique Cannon
On International Day of Women In Science, we’re proud to shine a spotlight on Orthocell’s Quality Director, Monique Cannon.
Quarterly Report – Period To December 2021
Orthocell (ASX $OCC) has today published its Quarterly Report for the period ended 31 December 2021.
RADIO INTERVIEW | Bulls N’ Bears Report
Orthocell (ASX $OCC) MD, Paul Anderson, was interviewed by Matt Birney on the Bulls N’ Bears Report about our latest OrthoATI™ clinical data.
The West Australian | Orthocell’s tendon injury treatment a game-changer, clinical trial finds
Orthocell (ASX $OCC) has been featured in The West Australian in a story titled, “Orthocell’s tendon injury treatment a game-changer, clinical trial finds”, following the release of positive clinical data for our OrthoATI™ cell therapy last week.
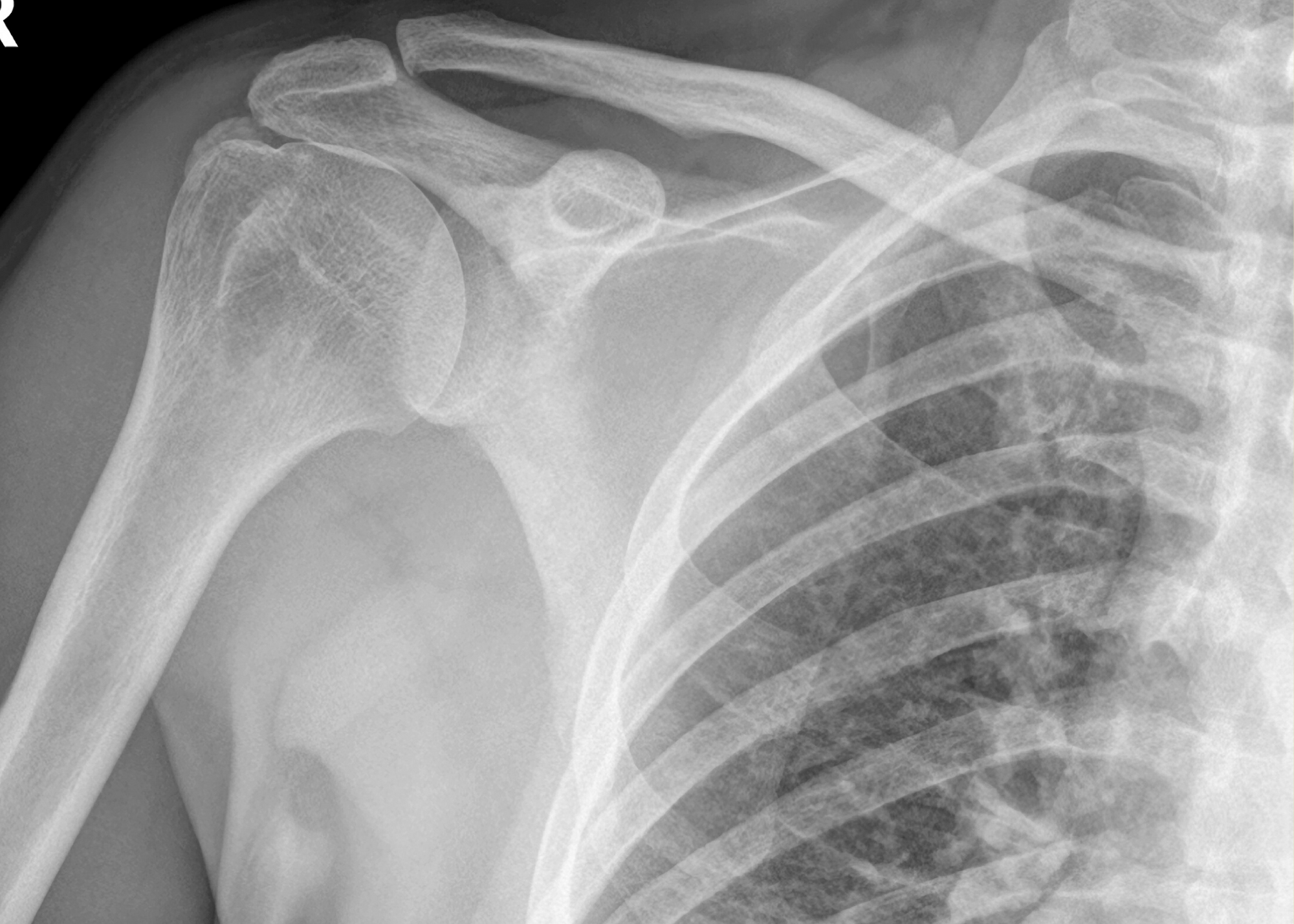
Positive clinical results for OrthoATI™
Orthocell (ASX $OCC) has today released positive clinical data for its OrthoATI™ tendon program, which shows that OrthoATI™ is statistically more effective than the current standard of care (corticosteroid injection) for treatment of rotator cuff tendinopathy with intrasubstance tendon tear.
Ortho-ATI® Update
Orthocell (ASX $OCC) has today released an update on its Ortho-ATI™ rotator cuff tendon study – the database is now locked and the Company is track to report the highly anticipated top line results in Q4 this calendar year.
This study was designed to assess the effectiveness of Ortho-ATI™, compared to corticosteroids, as a non-surgical treatment for a complex, hard-to-treat clinical problem.
Should the study meet its objectives, Orthocell is well positioned to deliver the first FDA approved injectable cell therapy in orthopaedics for the treatment of chronic tendon injuries in a rotator cuff market estimated at around US$2.8b per annum.
Orthocell featured as a Case Study in the Australian Regenerative Medicine Therapies Report
Orthocell’s CelGro™ technology has been featured as one of only four case studies in the recent Australian Regenerative Medicine Therapies report.
Investor Presentation | Canaccord Genuity South-West Connect ASX Showcase
Orthocell MD, Paul Anderson, presented at the Canaccord Genuity South-West Connect ASX Showcase today to share the latest news and developments for the Company’s Ortho-ATI™ and CelGro™ platform technologies.
Orthocell (ASX:OCC) announces positive data on CelGro collagen rope for ACL repair
Orthocell has been featured in The Market Herald, following our announcement of positive data from a pre-clinical study for anterior cruciate ligament (ACL) reconstruction.