Orthocell welcomed 9 News journalist Michael Stamp to Orthocell HQ last week to film an in-depth segment about our FDA clearance for the nightly news.
US FDA grants 510(k) clearance for Orthocell’s flagship Remplir™ nerve repair product
Orthocell has announced that the US Food and Drug Administration has granted regulatory clearance under the 510(k) pathway for the Company’s nerve repair product, Remplir™
First Sales of Striate+™ in DACH Region
Orthocell has today announced that our exclusive global distribution partner BioHorizons has officially completed first sales of Orthocell’s dental product, Striate+™, in Germany, Austria and Switzerland.
Orthocell Receives Regulatory Approval for Striate+ in Singapore
Orthocell has received regulatory approval from Singapore’s Health Sciences Authority (HSA) for its market leading dental guided bone regeneration product, Striate+™.
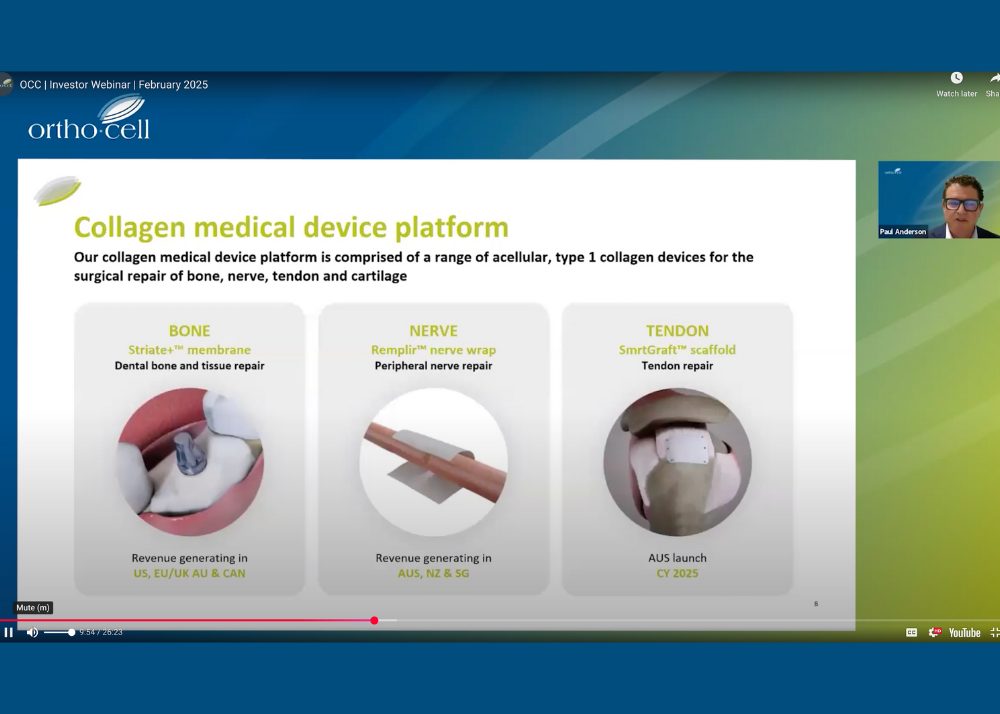
REPLAY | Investor Webinar
Orthocell CEO and MD, Paul Anderson, hosted a webinar this morning to provide an important update on Orthocell’s clinical and commercial plans.
Watch the replay:
Thailand Regulatory Application for Remplir™
Orthocell has submitted a regulatory application to the FDA of Thailand to commence sales of its groundbreaking nerve repair device Remplir™, with clearance expected in Q3 CY2025.
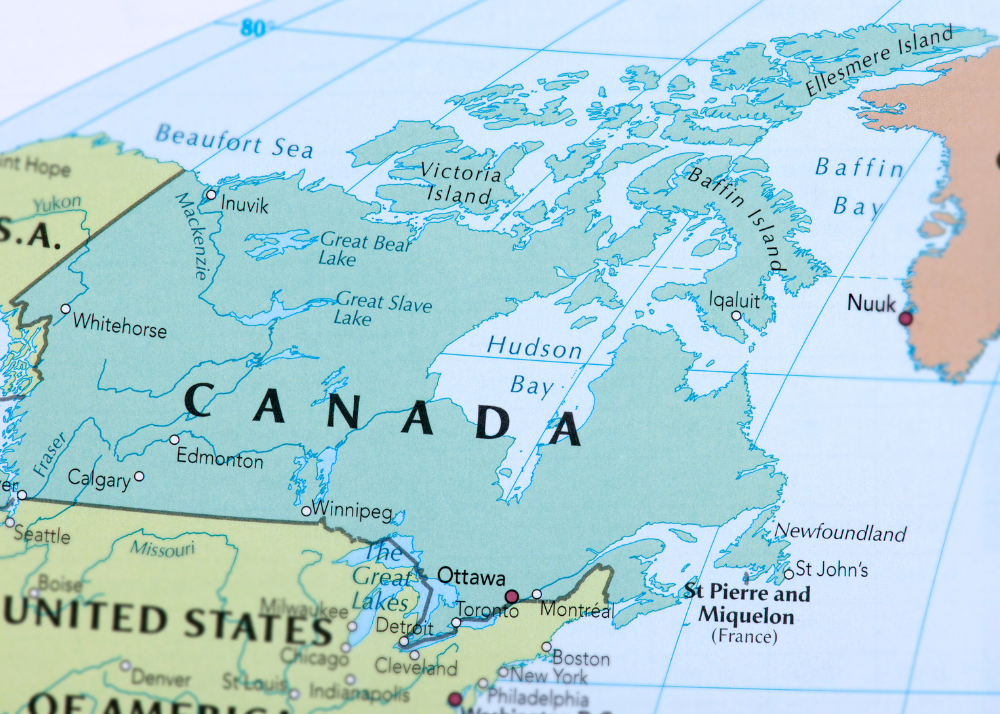
Orthocell Submits Canada Regulatory Application to Commence Sales of Remplir™ into US$75million Canadian market
Orthocell has announced it has submitted an application to Health Canada for a Medical Device Licence (MDL) to sell its leading nerve repair product Remplir™ into the US$75 million Canadian nerve repair market.
Stockhead | Orthocell soars 14% after crushing early sales growth for Remplir
Orthocell’s plans to broaden its commercial footprint in existing and new markets drove the company’s share price up 13.8% this week, according to a special report in Stockhead.
Orthocell Accelerates Global Expansion: Five New Country Regulatory Submissions Planned in 2025
On the back of outstanding early sales traction for Remplir™ in Australia, New Zealand and Singapore, Orthocell has announced that it is accelerating its global expansion into multiple key jurisdictions.
Orthocell receives $3.18m R&D tax incentive refund
Orthocell has announced that it has received a $3.18 million R&D Tax Incentive Refund for the 2023/2024 financial year.